
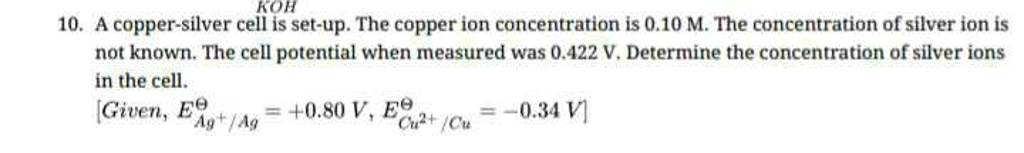
HULUSUL PURCULUI 13.A Copper-silver cell is up. The copper ion concentration in it is 0.10M.The concentration of silver is not known. The cell potential measured is 0.422V.Determine the concentration of silver ion

7. of A copper silver cell is setup. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measures 0.422V. Determine the
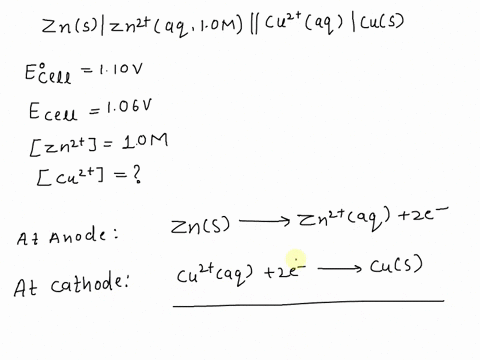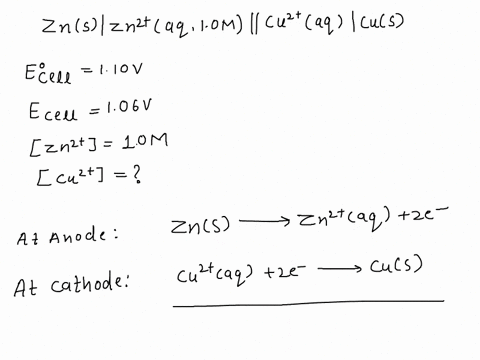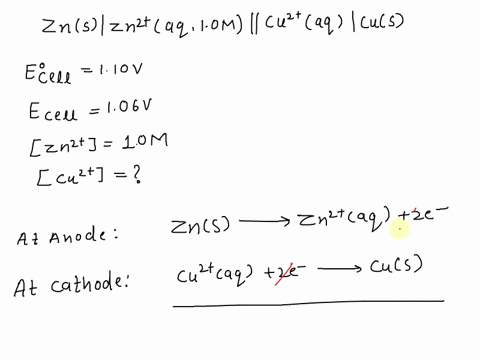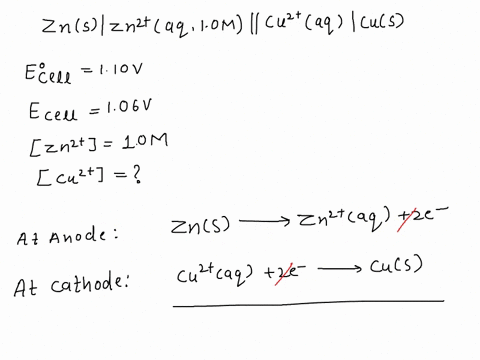
SOLVED: A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured as 0.422 V.

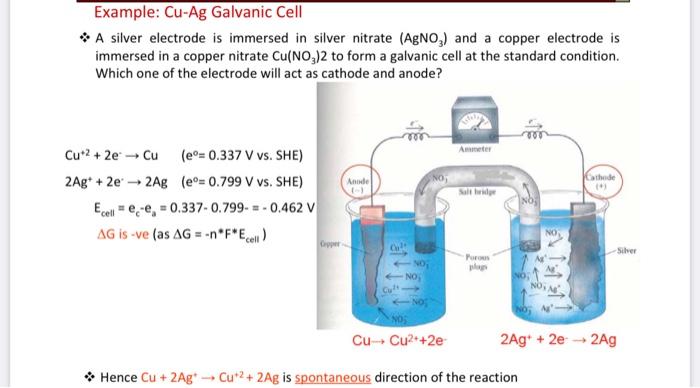
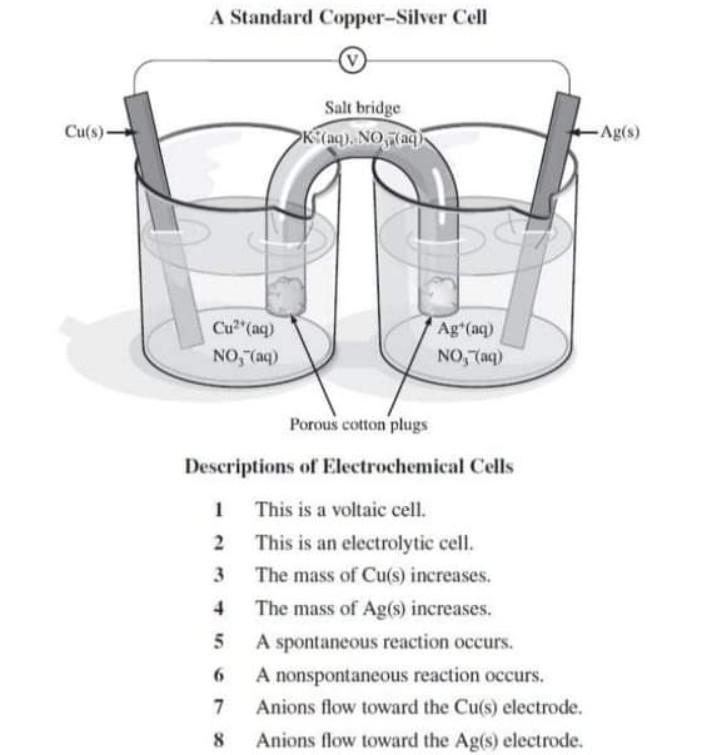
III. Cell Potentials Under Standard Conditions B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that includes all the components (

A copper - silver cell is set up. The copper ion concentrations is 0.10 M. The concentration of... - YouTube

The electrochemical cell for EMF measurement of chains of type (4.1)... | Download Scientific Diagram
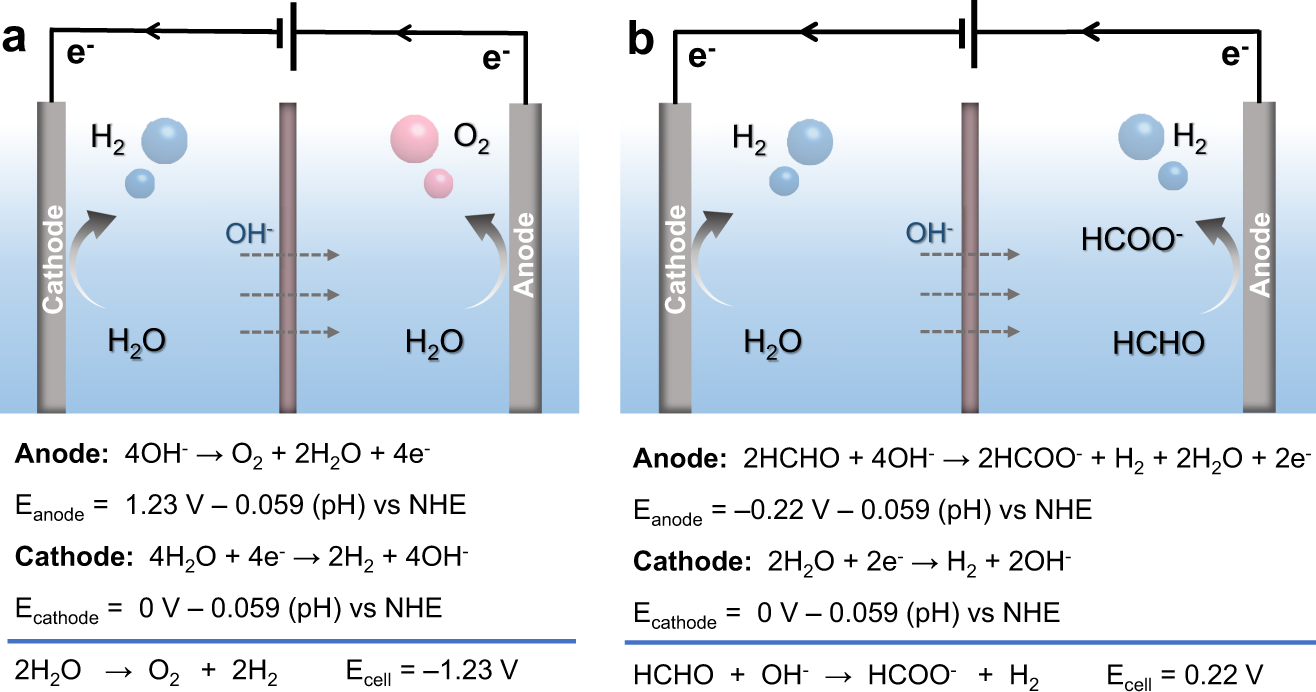
Dual hydrogen production from electrocatalytic water reduction coupled with formaldehyde oxidation via a copper-silver electrocatalyst | Nature Communications

Following cell is set up between copper and silver electrodes Cu//Cu^(2+)(aq)||Ag^(+)//Ag. If tw... - YouTube

A copper- silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measured 0.422 V. Determine the
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not know.the cell potential when measured was 0.422V determine the concentration of

13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in

13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in







![Assamese] A copper silver cell is set up. The copper ion concentrati Assamese] A copper silver cell is set up. The copper ion concentrati](https://static.doubtnut.com/ss/web-overlay-thumb/8484396.webp)